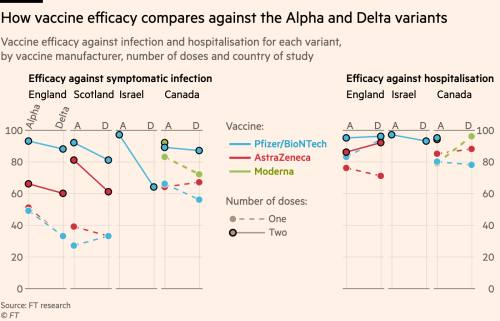
Collection of Astrazeneca vaccine efficacy ~ CHICAGO AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to long-awaited results of the companys US. Waiting three months between the first and second dose of the AstraZeneca COVID-19 vaccine results in high efficacy backing current recommendations.
as we know it recently is being searched by users around us, maybe one of you. Individuals are now accustomed to using the net in gadgets to see video and image information for inspiration, and according to the title of this article I will talk about about Astrazeneca Vaccine Efficacy In The Lancet Merryn Voysey and colleagues1 report the updated primary efficacy results for the OxfordAstraZeneca ChAdOx1 nCoV-19 AZD1222 vaccine from three single-blind randomised controlled trials in the UK and Brazil and one double-blind study in South Africa24 The ChAdOx1.
Astrazeneca vaccine efficacy
Collection of Astrazeneca vaccine efficacy ~ S1 1527 the overall. S1 1527 the overall. S1 1527 the overall. S1 1527 the overall. The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use. The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use. The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use. The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use. The interim analysis published today in The Lancet identified no severe coronavirus disease or hospitalizations in pooled results from the. The interim analysis published today in The Lancet identified no severe coronavirus disease or hospitalizations in pooled results from the. The interim analysis published today in The Lancet identified no severe coronavirus disease or hospitalizations in pooled results from the. The interim analysis published today in The Lancet identified no severe coronavirus disease or hospitalizations in pooled results from the.
The AZD1222 vaccine against COVID-19 has an efficacy of 6309 against symptomatic SARS-CoV-2 infection. The AZD1222 vaccine against COVID-19 has an efficacy of 6309 against symptomatic SARS-CoV-2 infection. The AZD1222 vaccine against COVID-19 has an efficacy of 6309 against symptomatic SARS-CoV-2 infection. The AZD1222 vaccine against COVID-19 has an efficacy of 6309 against symptomatic SARS-CoV-2 infection. This analysis includes data from four ongoing blinded randomised. This analysis includes data from four ongoing blinded randomised. This analysis includes data from four ongoing blinded randomised. This analysis includes data from four ongoing blinded randomised. The highly anticipated Covid-19 vaccine developed by the Oxford University with the pharmaceutical company AstraZeneca has been approved for use in the UK by the British regulator its a homegrown one this time. The highly anticipated Covid-19 vaccine developed by the Oxford University with the pharmaceutical company AstraZeneca has been approved for use in the UK by the British regulator its a homegrown one this time. The highly anticipated Covid-19 vaccine developed by the Oxford University with the pharmaceutical company AstraZeneca has been approved for use in the UK by the British regulator its a homegrown one this time. The highly anticipated Covid-19 vaccine developed by the Oxford University with the pharmaceutical company AstraZeneca has been approved for use in the UK by the British regulator its a homegrown one this time.
In addition weekly swabbing are done for detection of infection and assessment of vaccine efficacy against infection. In addition weekly swabbing are done for detection of infection and assessment of vaccine efficacy against infection. In addition weekly swabbing are done for detection of infection and assessment of vaccine efficacy against infection. In addition weekly swabbing are done for detection of infection and assessment of vaccine efficacy against infection. A safe and efficacious vaccine against severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 if deployed with high coverage could contribute to the control of the COVID-19 pandemic. A safe and efficacious vaccine against severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 if deployed with high coverage could contribute to the control of the COVID-19 pandemic. A safe and efficacious vaccine against severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 if deployed with high coverage could contribute to the control of the COVID-19 pandemic. A safe and efficacious vaccine against severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 if deployed with high coverage could contribute to the control of the COVID-19 pandemic. New vaccine efficacy results are reported now in The Lancet. New vaccine efficacy results are reported now in The Lancet. New vaccine efficacy results are reported now in The Lancet. New vaccine efficacy results are reported now in The Lancet.
The primary analysis of the Phase III clinical trials from the UK Brazil and South Africa published as a preprint in The Lancet confirmed COVID-19 Vaccine AstraZeneca is safe and effective at preventing COVID-19 with no severe cases and no hospitalisations more than 22 days after the first dose. The primary analysis of the Phase III clinical trials from the UK Brazil and South Africa published as a preprint in The Lancet confirmed COVID-19 Vaccine AstraZeneca is safe and effective at preventing COVID-19 with no severe cases and no hospitalisations more than 22 days after the first dose. The primary analysis of the Phase III clinical trials from the UK Brazil and South Africa published as a preprint in The Lancet confirmed COVID-19 Vaccine AstraZeneca is safe and effective at preventing COVID-19 with no severe cases and no hospitalisations more than 22 days after the first dose. The primary analysis of the Phase III clinical trials from the UK Brazil and South Africa published as a preprint in The Lancet confirmed COVID-19 Vaccine AstraZeneca is safe and effective at preventing COVID-19 with no severe cases and no hospitalisations more than 22 days after the first dose. The data showed the vaccine had an 835 estimated efficacy in. The data showed the vaccine had an 835 estimated efficacy in. The data showed the vaccine had an 835 estimated efficacy in. The data showed the vaccine had an 835 estimated efficacy in. Oxford-AstraZeneca COVID-19 vaccine efficacy Lancet. Oxford-AstraZeneca COVID-19 vaccine efficacy Lancet. Oxford-AstraZeneca COVID-19 vaccine efficacy Lancet. Oxford-AstraZeneca COVID-19 vaccine efficacy Lancet.
Does it work against new variants. Does it work against new variants. Does it work against new variants. Does it work against new variants. In this vulnerable group the. In this vulnerable group the. In this vulnerable group the. In this vulnerable group the. Clinical trial published on Wednesday. Clinical trial published on Wednesday. Clinical trial published on Wednesday. Clinical trial published on Wednesday.
We evaluated the safety and efficacy of the ChAdOx1 nCoV-19 vaccine in a pooled interim analysis of four trials. We evaluated the safety and efficacy of the ChAdOx1 nCoV-19 vaccine in a pooled interim analysis of four trials. We evaluated the safety and efficacy of the ChAdOx1 nCoV-19 vaccine in a pooled interim analysis of four trials. We evaluated the safety and efficacy of the ChAdOx1 nCoV-19 vaccine in a pooled interim analysis of four trials. The OxfordAstraZeneca COVID-19 vaccine codenamed AZD1222 and sold under the brand names Covishield and Vaxzevria among others is a viral vector vaccine for prevention of COVID-19Developed in the United Kingdom by the Oxford University and British-Swedish company AstraZeneca it is given by intramuscular injection using as a vector the modified chimpanzee adenovirus ChAdOx1. The OxfordAstraZeneca COVID-19 vaccine codenamed AZD1222 and sold under the brand names Covishield and Vaxzevria among others is a viral vector vaccine for prevention of COVID-19Developed in the United Kingdom by the Oxford University and British-Swedish company AstraZeneca it is given by intramuscular injection using as a vector the modified chimpanzee adenovirus ChAdOx1. The OxfordAstraZeneca COVID-19 vaccine codenamed AZD1222 and sold under the brand names Covishield and Vaxzevria among others is a viral vector vaccine for prevention of COVID-19Developed in the United Kingdom by the Oxford University and British-Swedish company AstraZeneca it is given by intramuscular injection using as a vector the modified chimpanzee adenovirus ChAdOx1. The OxfordAstraZeneca COVID-19 vaccine codenamed AZD1222 and sold under the brand names Covishield and Vaxzevria among others is a viral vector vaccine for prevention of COVID-19Developed in the United Kingdom by the Oxford University and British-Swedish company AstraZeneca it is given by intramuscular injection using as a vector the modified chimpanzee adenovirus ChAdOx1. COV003 is a single-blinded multi-centre randomised controlled Phase III trial assessing the safety efficacy and immunogenicity. COV003 is a single-blinded multi-centre randomised controlled Phase III trial assessing the safety efficacy and immunogenicity. COV003 is a single-blinded multi-centre randomised controlled Phase III trial assessing the safety efficacy and immunogenicity. COV003 is a single-blinded multi-centre randomised controlled Phase III trial assessing the safety efficacy and immunogenicity.
Oxford-AstraZeneca COVID-19 vaccine efficacy. Oxford-AstraZeneca COVID-19 vaccine efficacy. Oxford-AstraZeneca COVID-19 vaccine efficacy. Oxford-AstraZeneca COVID-19 vaccine efficacy. Results demonstrated vaccine efficacy of 76 CI. Results demonstrated vaccine efficacy of 76 CI. Results demonstrated vaccine efficacy of 76 CI. Results demonstrated vaccine efficacy of 76 CI. Investigators of four randomised controlled trials conducted in the UK South Africa and Brazil report pooled results of an interim analysis of safety and efficacy against COVID-19 of the OxfordAstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 AZD1222 in adults. Investigators of four randomised controlled trials conducted in the UK South Africa and Brazil report pooled results of an interim analysis of safety and efficacy against COVID-19 of the OxfordAstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 AZD1222 in adults. Investigators of four randomised controlled trials conducted in the UK South Africa and Brazil report pooled results of an interim analysis of safety and efficacy against COVID-19 of the OxfordAstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 AZD1222 in adults. Investigators of four randomised controlled trials conducted in the UK South Africa and Brazil report pooled results of an interim analysis of safety and efficacy against COVID-19 of the OxfordAstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 AZD1222 in adults.
Overall efficacy of 74 was lower than the interim 79 figure reported by the British drugmaker in March a result AstraZeneca. Overall efficacy of 74 was lower than the interim 79 figure reported by the British drugmaker in March a result AstraZeneca. Overall efficacy of 74 was lower than the interim 79 figure reported by the British drugmaker in March a result AstraZeneca. Overall efficacy of 74 was lower than the interim 79 figure reported by the British drugmaker in March a result AstraZeneca. Oxford-AstraZeneca COVID-19 vaccine efficacy. Oxford-AstraZeneca COVID-19 vaccine efficacy. Oxford-AstraZeneca COVID-19 vaccine efficacy. Oxford-AstraZeneca COVID-19 vaccine efficacy. People under the age of 40 are to be offered an alternative to the Oxford-AstraZeneca vaccine in the UK as a precaution after a review of all the latest evidence by. People under the age of 40 are to be offered an alternative to the Oxford-AstraZeneca vaccine in the UK as a precaution after a review of all the latest evidence by. People under the age of 40 are to be offered an alternative to the Oxford-AstraZeneca vaccine in the UK as a precaution after a review of all the latest evidence by. People under the age of 40 are to be offered an alternative to the Oxford-AstraZeneca vaccine in the UK as a precaution after a review of all the latest evidence by.
1 4 5 7 The ChAdOx1 nCoV-19 vaccine can also use routine refrigerated cold chain which is important since the ultra-low. 1 4 5 7 The ChAdOx1 nCoV-19 vaccine can also use routine refrigerated cold chain which is important since the ultra-low. 1 4 5 7 The ChAdOx1 nCoV-19 vaccine can also use routine refrigerated cold chain which is important since the ultra-low. 1 4 5 7 The ChAdOx1 nCoV-19 vaccine can also use routine refrigerated cold chain which is important since the ultra-low. In a post hoc analysis of vaccine efficacy at more than 14 days after a single injection through October 31 2020 as a proxy for infection by a nonB1351 variant Fig. In a post hoc analysis of vaccine efficacy at more than 14 days after a single injection through October 31 2020 as a proxy for infection by a nonB1351 variant Fig. In a post hoc analysis of vaccine efficacy at more than 14 days after a single injection through October 31 2020 as a proxy for infection by a nonB1351 variant Fig. In a post hoc analysis of vaccine efficacy at more than 14 days after a single injection through October 31 2020 as a proxy for infection by a nonB1351 variant Fig. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people.
CHICAGO Sept 30 AstraZeneca Plcs Covid-19 vaccine demonstrated 74 per cent efficacy at preventing symptomatic disease a figure that increased to 835 per cent in people aged 65 and older according to long-awaited results of the companys US clinical trial published on Wednesday. CHICAGO Sept 30 AstraZeneca Plcs Covid-19 vaccine demonstrated 74 per cent efficacy at preventing symptomatic disease a figure that increased to 835 per cent in people aged 65 and older according to long-awaited results of the companys US clinical trial published on Wednesday. CHICAGO Sept 30 AstraZeneca Plcs Covid-19 vaccine demonstrated 74 per cent efficacy at preventing symptomatic disease a figure that increased to 835 per cent in people aged 65 and older according to long-awaited results of the companys US clinical trial published on Wednesday. CHICAGO Sept 30 AstraZeneca Plcs Covid-19 vaccine demonstrated 74 per cent efficacy at preventing symptomatic disease a figure that increased to 835 per cent in people aged 65 and older according to long-awaited results of the companys US clinical trial published on Wednesday. The COVID-19 vaccine developed by AstraZeneca PLC and Oxford University showed 74 efficacy at preventing symptomatic illness in a large late-stage trial conducted in the US Chile and Peru. The COVID-19 vaccine developed by AstraZeneca PLC and Oxford University showed 74 efficacy at preventing symptomatic illness in a large late-stage trial conducted in the US Chile and Peru. The COVID-19 vaccine developed by AstraZeneca PLC and Oxford University showed 74 efficacy at preventing symptomatic illness in a large late-stage trial conducted in the US Chile and Peru. The COVID-19 vaccine developed by AstraZeneca PLC and Oxford University showed 74 efficacy at preventing symptomatic illness in a large late-stage trial conducted in the US Chile and Peru. The Anglo-Swedish firm has. The Anglo-Swedish firm has. The Anglo-Swedish firm has. The Anglo-Swedish firm has.
Sponsored by the US. Sponsored by the US. Sponsored by the US. Sponsored by the US. Longer dose intervals within the 8 to 12 weeks range are associated with greater vaccine efficacy. Longer dose intervals within the 8 to 12 weeks range are associated with greater vaccine efficacy. Longer dose intervals within the 8 to 12 weeks range are associated with greater vaccine efficacy. Longer dose intervals within the 8 to 12 weeks range are associated with greater vaccine efficacy. Government the new AstraZeneca trial in the United States Peru and Chile is the largest test yet of the vaccines worth and the only one in which a sizable chunk of the participants20was over 65 years old. Government the new AstraZeneca trial in the United States Peru and Chile is the largest test yet of the vaccines worth and the only one in which a sizable chunk of the participants20was over 65 years old. Government the new AstraZeneca trial in the United States Peru and Chile is the largest test yet of the vaccines worth and the only one in which a sizable chunk of the participants20was over 65 years old. Government the new AstraZeneca trial in the United States Peru and Chile is the largest test yet of the vaccines worth and the only one in which a sizable chunk of the participants20was over 65 years old.
SAGE has reviewed all available data on the performance of the vaccine in the settings of variants of concern. SAGE has reviewed all available data on the performance of the vaccine in the settings of variants of concern. SAGE has reviewed all available data on the performance of the vaccine in the settings of variants of concern. SAGE has reviewed all available data on the performance of the vaccine in the settings of variants of concern. VACCINE efficacy is a confusing term which has become common language since the coronavirus pandemic began more than a year ago but what protection does the Oxford-AstraZeneca vaccine really give. VACCINE efficacy is a confusing term which has become common language since the coronavirus pandemic began more than a year ago but what protection does the Oxford-AstraZeneca vaccine really give. VACCINE efficacy is a confusing term which has become common language since the coronavirus pandemic began more than a year ago but what protection does the Oxford-AstraZeneca vaccine really give. VACCINE efficacy is a confusing term which has become common language since the coronavirus pandemic began more than a year ago but what protection does the Oxford-AstraZeneca vaccine really give. AstraZeneca has updated the efficacy result of its coronavirus vaccine trial in the US after health officials insisted they wanted to include the latest information. AstraZeneca has updated the efficacy result of its coronavirus vaccine trial in the US after health officials insisted they wanted to include the latest information. AstraZeneca has updated the efficacy result of its coronavirus vaccine trial in the US after health officials insisted they wanted to include the latest information. AstraZeneca has updated the efficacy result of its coronavirus vaccine trial in the US after health officials insisted they wanted to include the latest information.
The government has ordered 100 million doses by the end of March 2021. The government has ordered 100 million doses by the end of March 2021. The government has ordered 100 million doses by the end of March 2021. The government has ordered 100 million doses by the end of March 2021. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to. Study reveals PfizerBioNTech and OxfordAstraZeneca COVID-19 vaccine efficacy and immune response in clinically vulnerable individuals Download PDF Copy By Suchandrima Bhowmik Sep 6 2021 Reviewed. Study reveals PfizerBioNTech and OxfordAstraZeneca COVID-19 vaccine efficacy and immune response in clinically vulnerable individuals Download PDF Copy By Suchandrima Bhowmik Sep 6 2021 Reviewed. Study reveals PfizerBioNTech and OxfordAstraZeneca COVID-19 vaccine efficacy and immune response in clinically vulnerable individuals Download PDF Copy By Suchandrima Bhowmik Sep 6 2021 Reviewed. Study reveals PfizerBioNTech and OxfordAstraZeneca COVID-19 vaccine efficacy and immune response in clinically vulnerable individuals Download PDF Copy By Suchandrima Bhowmik Sep 6 2021 Reviewed.
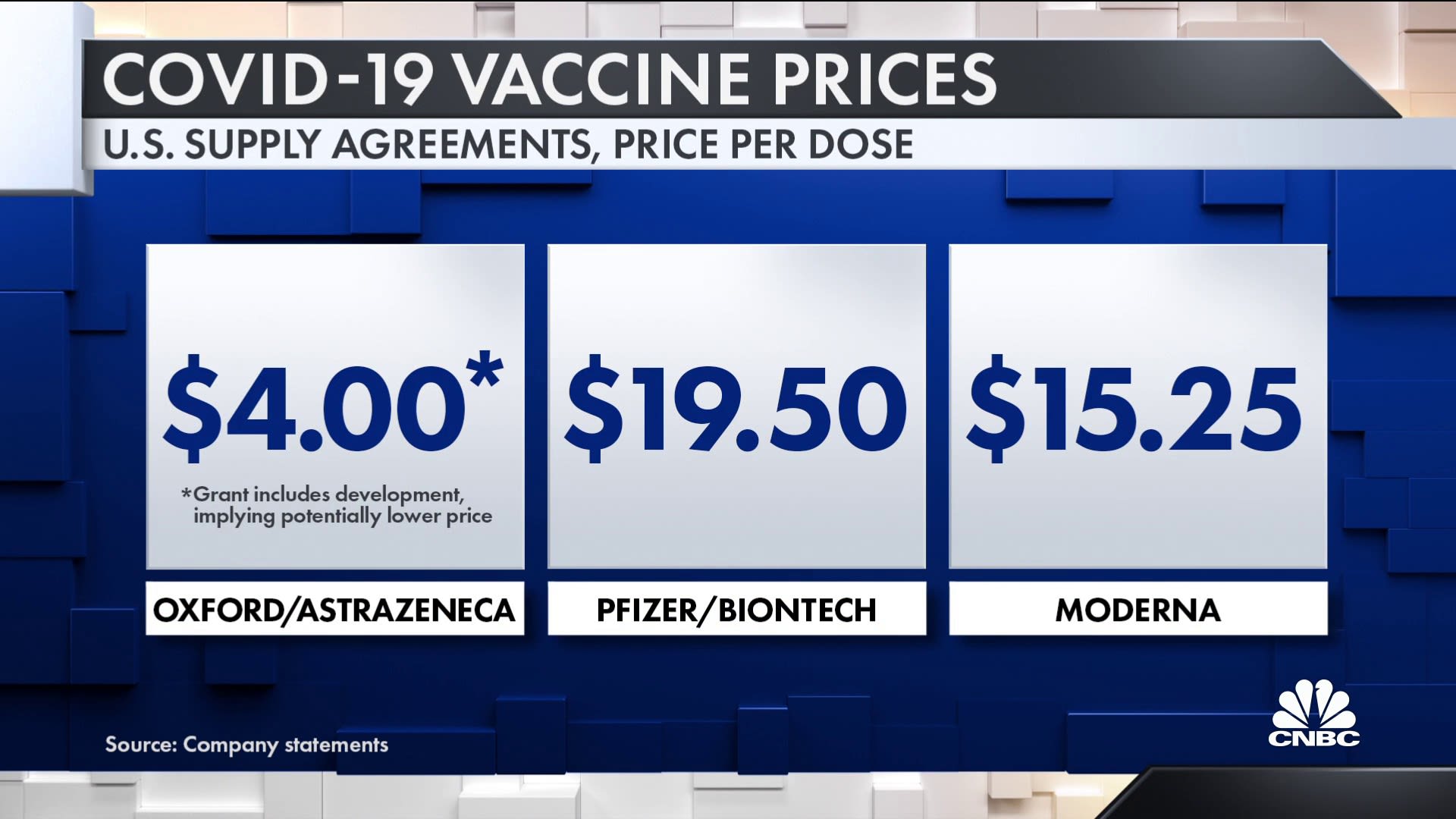
59 to 86 after a first dose with. 59 to 86 after a first dose with. 59 to 86 after a first dose with. 59 to 86 after a first dose with. Epub 2020 Dec 8. Epub 2020 Dec 8. Epub 2020 Dec 8. Epub 2020 Dec 8. OxfordAstraZenecas US23 per dose agreement with the COVAX Facility holds good promise for equitable access for LMICs compared with the high cost of the two mRNA vaccines that have reported more than 90 efficacy. OxfordAstraZenecas US23 per dose agreement with the COVAX Facility holds good promise for equitable access for LMICs compared with the high cost of the two mRNA vaccines that have reported more than 90 efficacy. OxfordAstraZenecas US23 per dose agreement with the COVAX Facility holds good promise for equitable access for LMICs compared with the high cost of the two mRNA vaccines that have reported more than 90 efficacy. OxfordAstraZenecas US23 per dose agreement with the COVAX Facility holds good promise for equitable access for LMICs compared with the high cost of the two mRNA vaccines that have reported more than 90 efficacy.
CHICAGO AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to long. CHICAGO AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to long. CHICAGO AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to long. CHICAGO AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to long. The AstraZeneca vaccine has been widely used in the UK. The AstraZeneca vaccine has been widely used in the UK. The AstraZeneca vaccine has been widely used in the UK. The AstraZeneca vaccine has been widely used in the UK. Authors Maria Deloria Knoll 1. Authors Maria Deloria Knoll 1. Authors Maria Deloria Knoll 1. Authors Maria Deloria Knoll 1.
And Europe but is not yet authorized in the US. And Europe but is not yet authorized in the US. And Europe but is not yet authorized in the US. And Europe but is not yet authorized in the US. Vaccines to prevent COVID-19 infection are crucial for an effective global pandemic response. Vaccines to prevent COVID-19 infection are crucial for an effective global pandemic response. Vaccines to prevent COVID-19 infection are crucial for an effective global pandemic response. Vaccines to prevent COVID-19 infection are crucial for an effective global pandemic response.
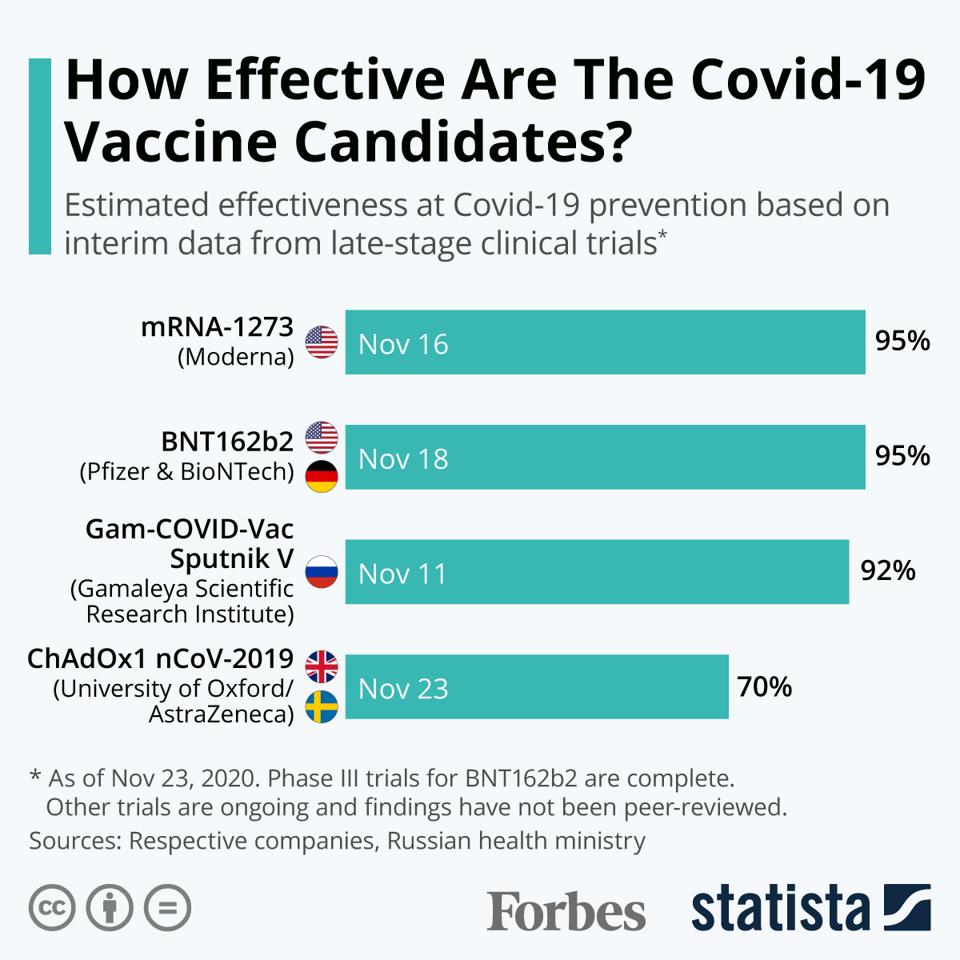
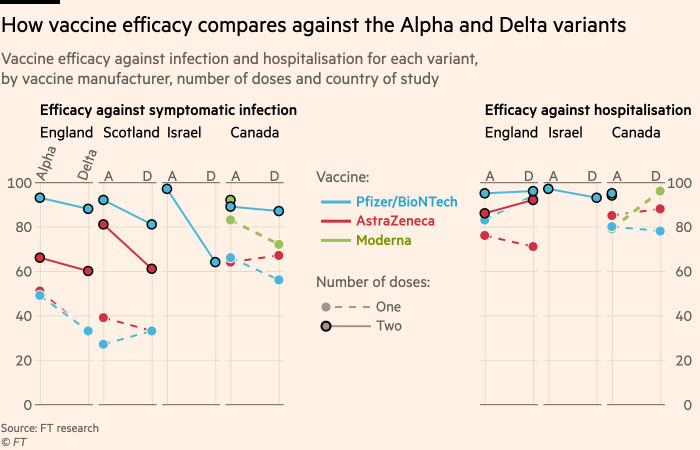
Chart How Effective Are Covid Vaccines At Preventing Infection Statista
Source Image @ www.statista.com
Astrazeneca vaccine efficacy | Chart How Effective Are Covid Vaccines At Preventing Infection Statista
Collection of Astrazeneca vaccine efficacy ~ S1 1527 the overall. S1 1527 the overall. S1 1527 the overall. The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use. The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use. The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use. The interim analysis published today in The Lancet identified no severe coronavirus disease or hospitalizations in pooled results from the. The interim analysis published today in The Lancet identified no severe coronavirus disease or hospitalizations in pooled results from the. The interim analysis published today in The Lancet identified no severe coronavirus disease or hospitalizations in pooled results from the.
The AZD1222 vaccine against COVID-19 has an efficacy of 6309 against symptomatic SARS-CoV-2 infection. The AZD1222 vaccine against COVID-19 has an efficacy of 6309 against symptomatic SARS-CoV-2 infection. The AZD1222 vaccine against COVID-19 has an efficacy of 6309 against symptomatic SARS-CoV-2 infection. This analysis includes data from four ongoing blinded randomised. This analysis includes data from four ongoing blinded randomised. This analysis includes data from four ongoing blinded randomised. The highly anticipated Covid-19 vaccine developed by the Oxford University with the pharmaceutical company AstraZeneca has been approved for use in the UK by the British regulator its a homegrown one this time. The highly anticipated Covid-19 vaccine developed by the Oxford University with the pharmaceutical company AstraZeneca has been approved for use in the UK by the British regulator its a homegrown one this time. The highly anticipated Covid-19 vaccine developed by the Oxford University with the pharmaceutical company AstraZeneca has been approved for use in the UK by the British regulator its a homegrown one this time.
In addition weekly swabbing are done for detection of infection and assessment of vaccine efficacy against infection. In addition weekly swabbing are done for detection of infection and assessment of vaccine efficacy against infection. In addition weekly swabbing are done for detection of infection and assessment of vaccine efficacy against infection. A safe and efficacious vaccine against severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 if deployed with high coverage could contribute to the control of the COVID-19 pandemic. A safe and efficacious vaccine against severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 if deployed with high coverage could contribute to the control of the COVID-19 pandemic. A safe and efficacious vaccine against severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 if deployed with high coverage could contribute to the control of the COVID-19 pandemic. New vaccine efficacy results are reported now in The Lancet. New vaccine efficacy results are reported now in The Lancet. New vaccine efficacy results are reported now in The Lancet.
The primary analysis of the Phase III clinical trials from the UK Brazil and South Africa published as a preprint in The Lancet confirmed COVID-19 Vaccine AstraZeneca is safe and effective at preventing COVID-19 with no severe cases and no hospitalisations more than 22 days after the first dose. The primary analysis of the Phase III clinical trials from the UK Brazil and South Africa published as a preprint in The Lancet confirmed COVID-19 Vaccine AstraZeneca is safe and effective at preventing COVID-19 with no severe cases and no hospitalisations more than 22 days after the first dose. The primary analysis of the Phase III clinical trials from the UK Brazil and South Africa published as a preprint in The Lancet confirmed COVID-19 Vaccine AstraZeneca is safe and effective at preventing COVID-19 with no severe cases and no hospitalisations more than 22 days after the first dose. The data showed the vaccine had an 835 estimated efficacy in. The data showed the vaccine had an 835 estimated efficacy in. The data showed the vaccine had an 835 estimated efficacy in. Oxford-AstraZeneca COVID-19 vaccine efficacy Lancet. Oxford-AstraZeneca COVID-19 vaccine efficacy Lancet. Oxford-AstraZeneca COVID-19 vaccine efficacy Lancet.
Does it work against new variants. Does it work against new variants. Does it work against new variants. In this vulnerable group the. In this vulnerable group the. In this vulnerable group the. Clinical trial published on Wednesday. Clinical trial published on Wednesday. Clinical trial published on Wednesday.
We evaluated the safety and efficacy of the ChAdOx1 nCoV-19 vaccine in a pooled interim analysis of four trials. We evaluated the safety and efficacy of the ChAdOx1 nCoV-19 vaccine in a pooled interim analysis of four trials. We evaluated the safety and efficacy of the ChAdOx1 nCoV-19 vaccine in a pooled interim analysis of four trials. The OxfordAstraZeneca COVID-19 vaccine codenamed AZD1222 and sold under the brand names Covishield and Vaxzevria among others is a viral vector vaccine for prevention of COVID-19Developed in the United Kingdom by the Oxford University and British-Swedish company AstraZeneca it is given by intramuscular injection using as a vector the modified chimpanzee adenovirus ChAdOx1. The OxfordAstraZeneca COVID-19 vaccine codenamed AZD1222 and sold under the brand names Covishield and Vaxzevria among others is a viral vector vaccine for prevention of COVID-19Developed in the United Kingdom by the Oxford University and British-Swedish company AstraZeneca it is given by intramuscular injection using as a vector the modified chimpanzee adenovirus ChAdOx1. The OxfordAstraZeneca COVID-19 vaccine codenamed AZD1222 and sold under the brand names Covishield and Vaxzevria among others is a viral vector vaccine for prevention of COVID-19Developed in the United Kingdom by the Oxford University and British-Swedish company AstraZeneca it is given by intramuscular injection using as a vector the modified chimpanzee adenovirus ChAdOx1. COV003 is a single-blinded multi-centre randomised controlled Phase III trial assessing the safety efficacy and immunogenicity. COV003 is a single-blinded multi-centre randomised controlled Phase III trial assessing the safety efficacy and immunogenicity. COV003 is a single-blinded multi-centre randomised controlled Phase III trial assessing the safety efficacy and immunogenicity.
Oxford-AstraZeneca COVID-19 vaccine efficacy. Oxford-AstraZeneca COVID-19 vaccine efficacy. Oxford-AstraZeneca COVID-19 vaccine efficacy. Results demonstrated vaccine efficacy of 76 CI. Results demonstrated vaccine efficacy of 76 CI. Results demonstrated vaccine efficacy of 76 CI. Investigators of four randomised controlled trials conducted in the UK South Africa and Brazil report pooled results of an interim analysis of safety and efficacy against COVID-19 of the OxfordAstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 AZD1222 in adults. Investigators of four randomised controlled trials conducted in the UK South Africa and Brazil report pooled results of an interim analysis of safety and efficacy against COVID-19 of the OxfordAstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 AZD1222 in adults. Investigators of four randomised controlled trials conducted in the UK South Africa and Brazil report pooled results of an interim analysis of safety and efficacy against COVID-19 of the OxfordAstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 AZD1222 in adults.
Overall efficacy of 74 was lower than the interim 79 figure reported by the British drugmaker in March a result AstraZeneca. Overall efficacy of 74 was lower than the interim 79 figure reported by the British drugmaker in March a result AstraZeneca. Overall efficacy of 74 was lower than the interim 79 figure reported by the British drugmaker in March a result AstraZeneca. Oxford-AstraZeneca COVID-19 vaccine efficacy. Oxford-AstraZeneca COVID-19 vaccine efficacy. Oxford-AstraZeneca COVID-19 vaccine efficacy. People under the age of 40 are to be offered an alternative to the Oxford-AstraZeneca vaccine in the UK as a precaution after a review of all the latest evidence by. People under the age of 40 are to be offered an alternative to the Oxford-AstraZeneca vaccine in the UK as a precaution after a review of all the latest evidence by. People under the age of 40 are to be offered an alternative to the Oxford-AstraZeneca vaccine in the UK as a precaution after a review of all the latest evidence by.
1 4 5 7 The ChAdOx1 nCoV-19 vaccine can also use routine refrigerated cold chain which is important since the ultra-low. 1 4 5 7 The ChAdOx1 nCoV-19 vaccine can also use routine refrigerated cold chain which is important since the ultra-low. 1 4 5 7 The ChAdOx1 nCoV-19 vaccine can also use routine refrigerated cold chain which is important since the ultra-low. In a post hoc analysis of vaccine efficacy at more than 14 days after a single injection through October 31 2020 as a proxy for infection by a nonB1351 variant Fig. In a post hoc analysis of vaccine efficacy at more than 14 days after a single injection through October 31 2020 as a proxy for infection by a nonB1351 variant Fig. In a post hoc analysis of vaccine efficacy at more than 14 days after a single injection through October 31 2020 as a proxy for infection by a nonB1351 variant Fig. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people.
CHICAGO Sept 30 AstraZeneca Plcs Covid-19 vaccine demonstrated 74 per cent efficacy at preventing symptomatic disease a figure that increased to 835 per cent in people aged 65 and older according to long-awaited results of the companys US clinical trial published on Wednesday. CHICAGO Sept 30 AstraZeneca Plcs Covid-19 vaccine demonstrated 74 per cent efficacy at preventing symptomatic disease a figure that increased to 835 per cent in people aged 65 and older according to long-awaited results of the companys US clinical trial published on Wednesday. CHICAGO Sept 30 AstraZeneca Plcs Covid-19 vaccine demonstrated 74 per cent efficacy at preventing symptomatic disease a figure that increased to 835 per cent in people aged 65 and older according to long-awaited results of the companys US clinical trial published on Wednesday. The COVID-19 vaccine developed by AstraZeneca PLC and Oxford University showed 74 efficacy at preventing symptomatic illness in a large late-stage trial conducted in the US Chile and Peru. The COVID-19 vaccine developed by AstraZeneca PLC and Oxford University showed 74 efficacy at preventing symptomatic illness in a large late-stage trial conducted in the US Chile and Peru. The COVID-19 vaccine developed by AstraZeneca PLC and Oxford University showed 74 efficacy at preventing symptomatic illness in a large late-stage trial conducted in the US Chile and Peru. The Anglo-Swedish firm has. The Anglo-Swedish firm has. The Anglo-Swedish firm has.
Sponsored by the US. Sponsored by the US. Sponsored by the US. Longer dose intervals within the 8 to 12 weeks range are associated with greater vaccine efficacy. Longer dose intervals within the 8 to 12 weeks range are associated with greater vaccine efficacy. Longer dose intervals within the 8 to 12 weeks range are associated with greater vaccine efficacy. Government the new AstraZeneca trial in the United States Peru and Chile is the largest test yet of the vaccines worth and the only one in which a sizable chunk of the participants20was over 65 years old. Government the new AstraZeneca trial in the United States Peru and Chile is the largest test yet of the vaccines worth and the only one in which a sizable chunk of the participants20was over 65 years old. Government the new AstraZeneca trial in the United States Peru and Chile is the largest test yet of the vaccines worth and the only one in which a sizable chunk of the participants20was over 65 years old.
SAGE has reviewed all available data on the performance of the vaccine in the settings of variants of concern. SAGE has reviewed all available data on the performance of the vaccine in the settings of variants of concern. SAGE has reviewed all available data on the performance of the vaccine in the settings of variants of concern. VACCINE efficacy is a confusing term which has become common language since the coronavirus pandemic began more than a year ago but what protection does the Oxford-AstraZeneca vaccine really give. VACCINE efficacy is a confusing term which has become common language since the coronavirus pandemic began more than a year ago but what protection does the Oxford-AstraZeneca vaccine really give. VACCINE efficacy is a confusing term which has become common language since the coronavirus pandemic began more than a year ago but what protection does the Oxford-AstraZeneca vaccine really give. AstraZeneca has updated the efficacy result of its coronavirus vaccine trial in the US after health officials insisted they wanted to include the latest information. AstraZeneca has updated the efficacy result of its coronavirus vaccine trial in the US after health officials insisted they wanted to include the latest information. AstraZeneca has updated the efficacy result of its coronavirus vaccine trial in the US after health officials insisted they wanted to include the latest information.
The government has ordered 100 million doses by the end of March 2021. The government has ordered 100 million doses by the end of March 2021. The government has ordered 100 million doses by the end of March 2021. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to. AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to. Study reveals PfizerBioNTech and OxfordAstraZeneca COVID-19 vaccine efficacy and immune response in clinically vulnerable individuals Download PDF Copy By Suchandrima Bhowmik Sep 6 2021 Reviewed. Study reveals PfizerBioNTech and OxfordAstraZeneca COVID-19 vaccine efficacy and immune response in clinically vulnerable individuals Download PDF Copy By Suchandrima Bhowmik Sep 6 2021 Reviewed. Study reveals PfizerBioNTech and OxfordAstraZeneca COVID-19 vaccine efficacy and immune response in clinically vulnerable individuals Download PDF Copy By Suchandrima Bhowmik Sep 6 2021 Reviewed.
59 to 86 after a first dose with. 59 to 86 after a first dose with. 59 to 86 after a first dose with. Epub 2020 Dec 8. Epub 2020 Dec 8. Epub 2020 Dec 8. OxfordAstraZenecas US23 per dose agreement with the COVAX Facility holds good promise for equitable access for LMICs compared with the high cost of the two mRNA vaccines that have reported more than 90 efficacy. OxfordAstraZenecas US23 per dose agreement with the COVAX Facility holds good promise for equitable access for LMICs compared with the high cost of the two mRNA vaccines that have reported more than 90 efficacy. OxfordAstraZenecas US23 per dose agreement with the COVAX Facility holds good promise for equitable access for LMICs compared with the high cost of the two mRNA vaccines that have reported more than 90 efficacy.
CHICAGO AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to long. CHICAGO AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to long. CHICAGO AstraZeneca Plcs COVID-19 vaccine demonstrated 74 efficacy at preventing symptomatic disease a figure that increased to 835 in people aged 65 and older according to long. The AstraZeneca vaccine has been widely used in the UK. The AstraZeneca vaccine has been widely used in the UK. The AstraZeneca vaccine has been widely used in the UK. Authors Maria Deloria Knoll 1. Authors Maria Deloria Knoll 1. Authors Maria Deloria Knoll 1.
If you re looking for Astrazeneca Vaccine Efficacy you've arrived at the ideal location. We ve got 20 images about astrazeneca vaccine efficacy including images, photos, photographs, backgrounds, and much more. In such web page, we additionally have variety of graphics available. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.

The Best Vaccine Don T Be Misled In Comparing Efficacy Of Johnson And Johnson Moderna And Pfizer Biontech Vox
Source Image @ www.vox.com

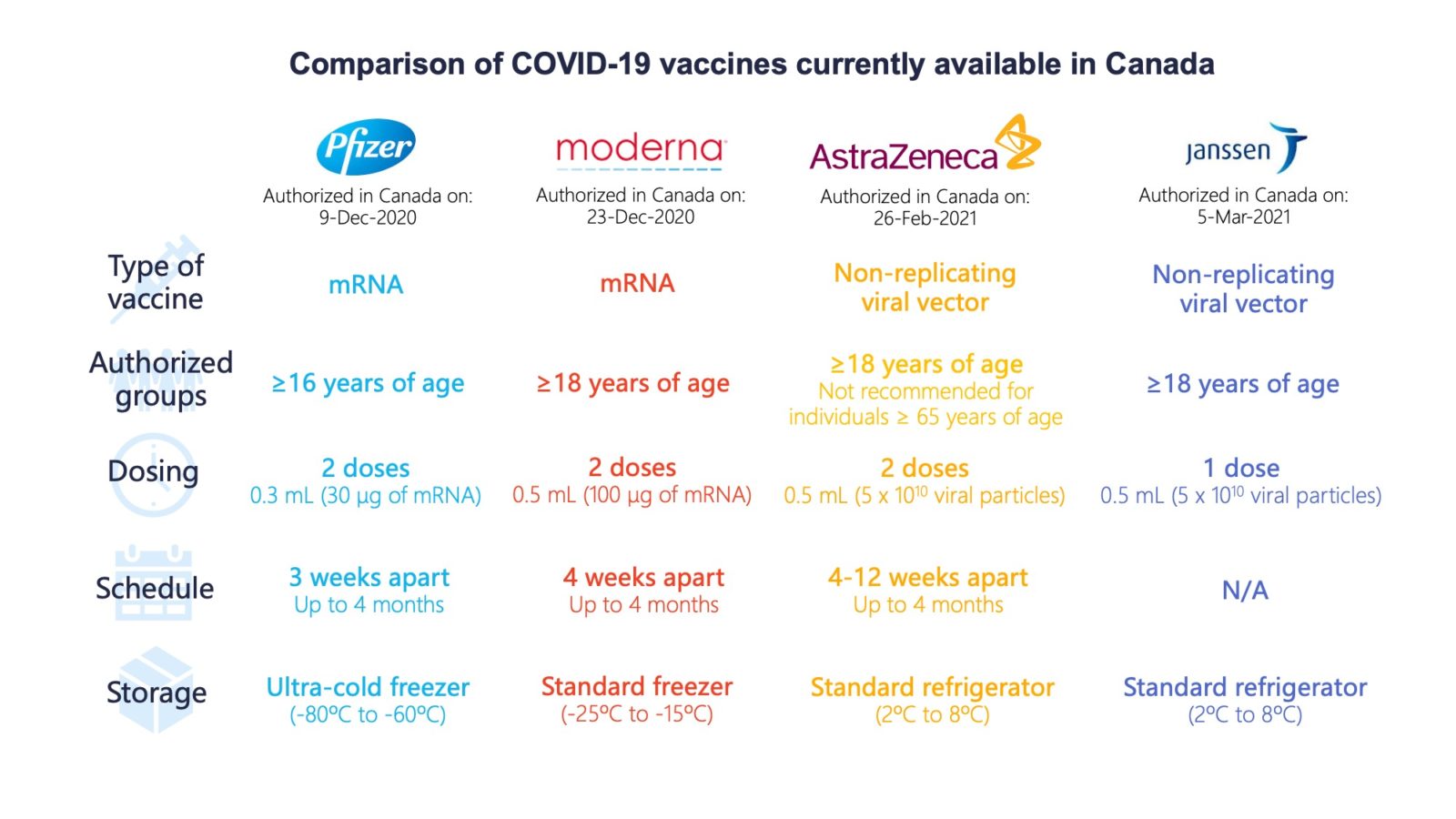
Explainer The Confusion Of Covishield Dosing
Source Image @ www.livemint.com
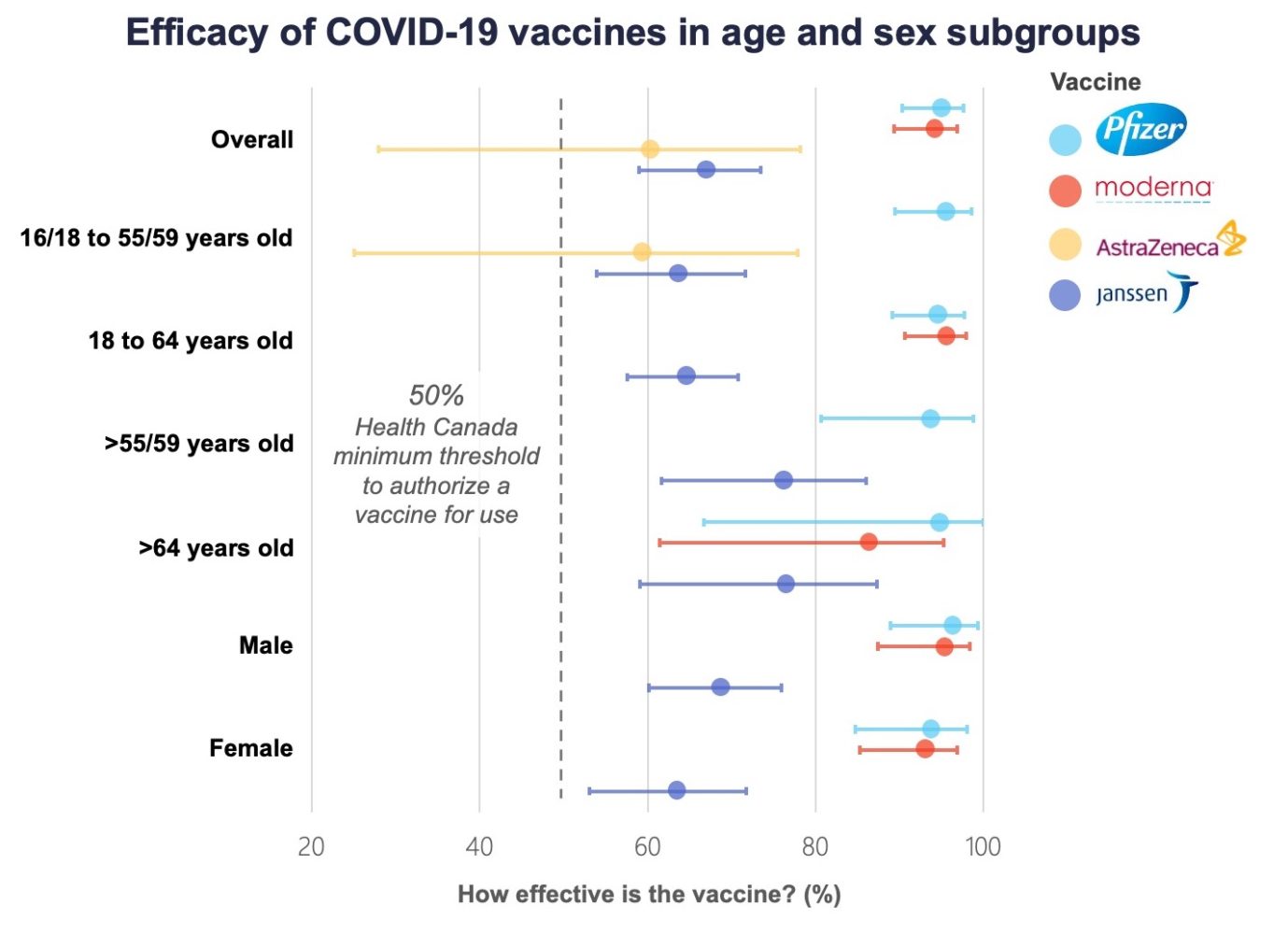
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate
Source Image @ healthydebate.ca
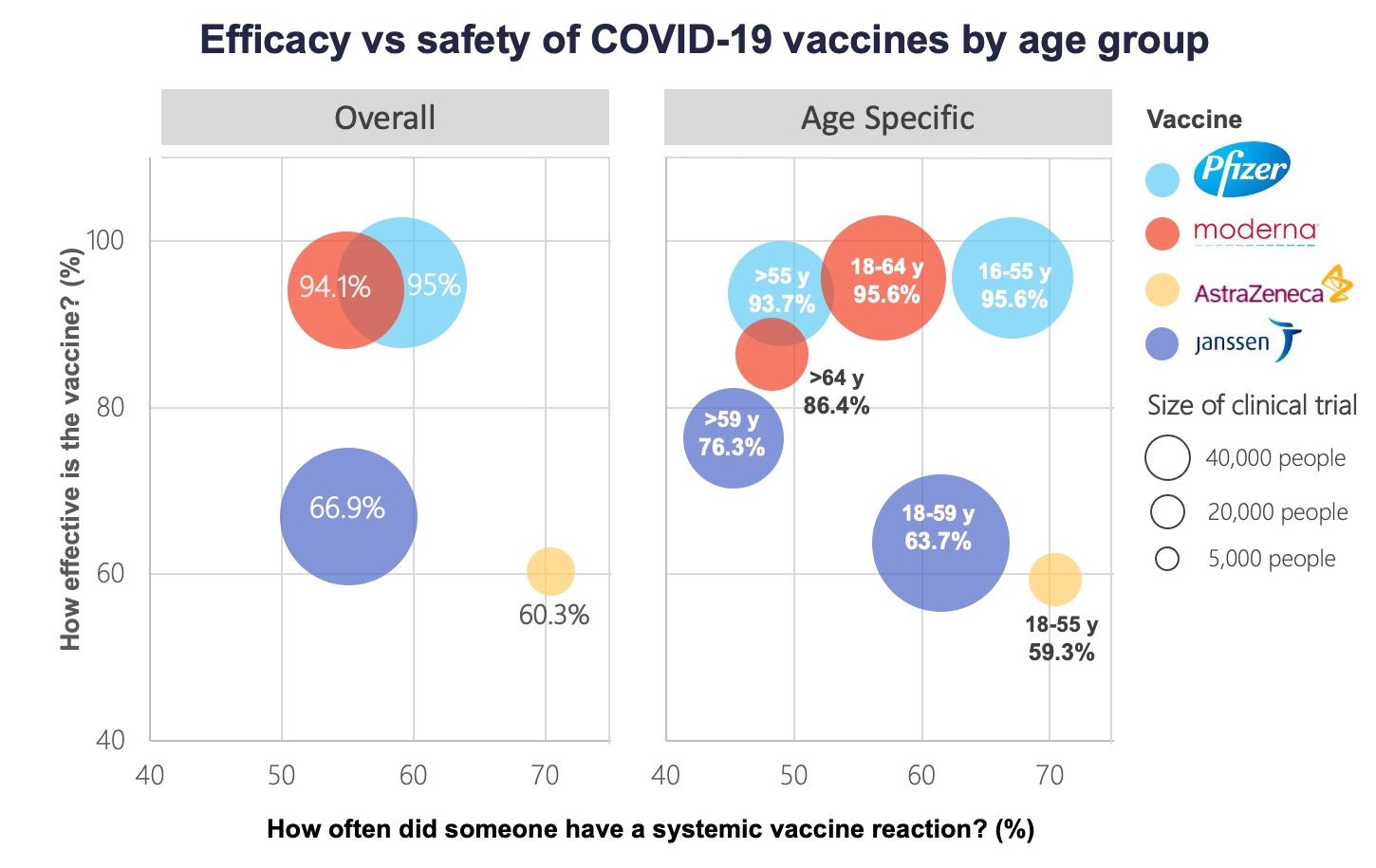
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate
Source Image @ healthydebate.ca